RoslinCT Boston
97 South St.
Hopkinton, MA
USA
RoslinCT Boston

Our global facilities across two continents (North America and Europe) are purposely designed to deliver high-quality, cell-based therapy products with our partners from development to clinical and commercial manufacturing.

Empowering your cell therapy breakthroughs, globally:
While designing the facilities, RoslinCT’s experienced team, considered all aspects of cell therapy operations, including people, process, and material flow, in compliance with global regulatory requirements.
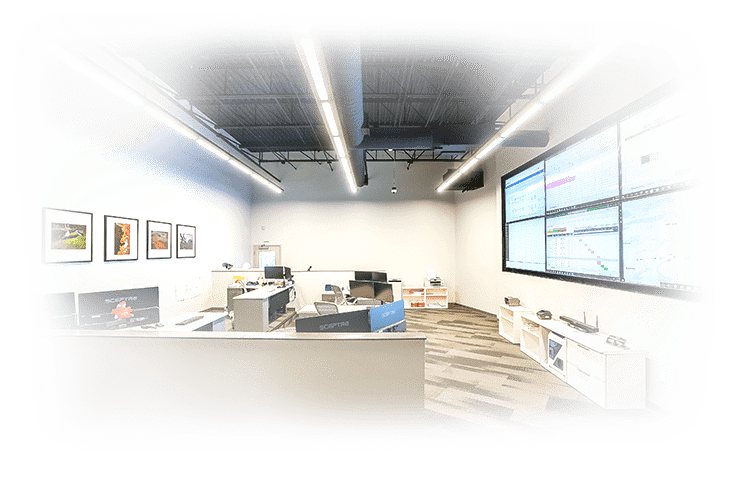
The RoslinCT Boston Operations Command Center monitors logistics and production activities in real-time, while sophisticated software platforms facilitate data transfer, reduce errors, and enable electronic batch records for real-time testing and product release.
Building a better future, one cell therapy at a time with our partners
Building or buying manufacturing capacity is a foundational decision. Years prior to reaching commercialization, a choice must be made.